Sports Medicine
Motion regained. Life renewed.
We are driven to lead the advancement of sports medicine with you.

Featured Sports Medicine products
Iconix Knotless all-suture knotless anchor
The 1.4mm Iconix Knotless implants are the smallest knotless all-suture implant on the market.1
Learn moreIconix HA+
A hydroxyapatite and bioglass coated all-suture anchor, designed to create the potential for accelerated bony ingrowth.
Learn moreInSpace
InSpace is the industry’s only minimally invasive biodegradable, subacromial balloon spacer for arthroscopic treatment of massive, irreparable rotator cuff tears (MIRCTs)†.
Learn more
We deliver
Stryker's Sports Medicine business is driven to advance sports medicine with you. We are a leading sports medicine company that delivers an integrated sports medicine product portfolio inclusive of the latest technology for arthroscopic and open shoulder, hip, knee and small joint procedures.
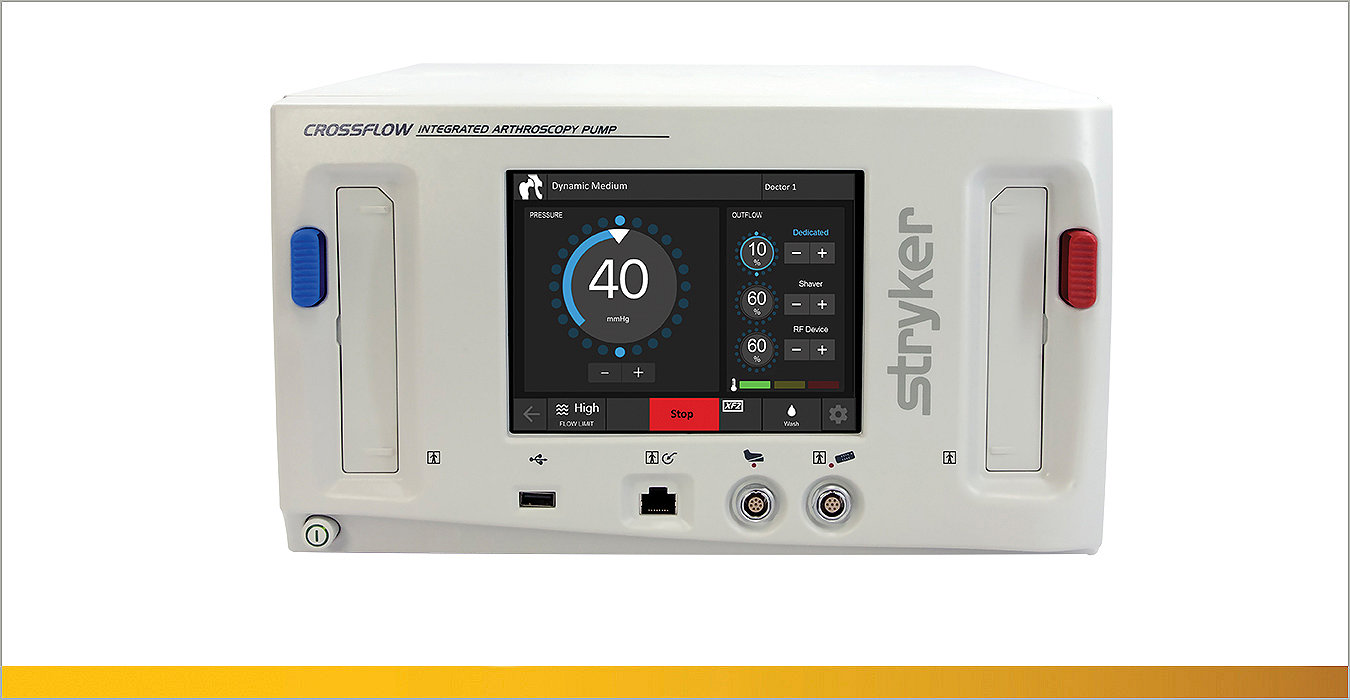
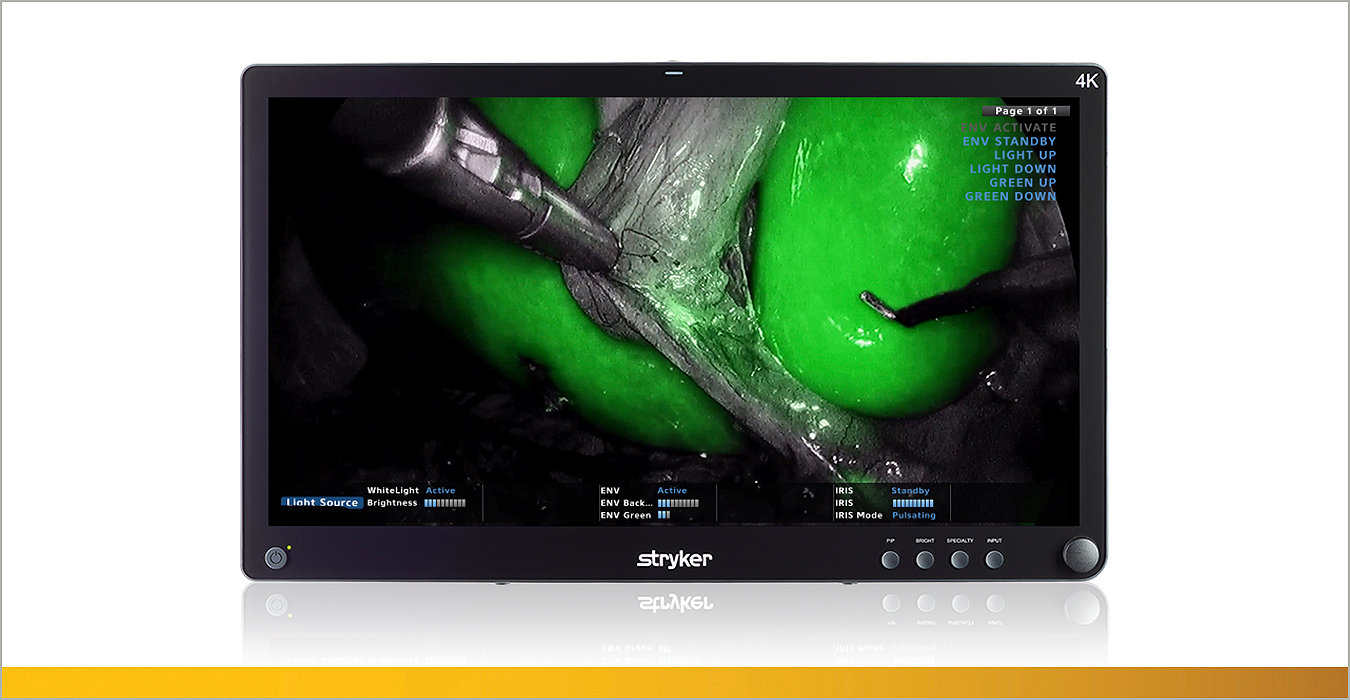
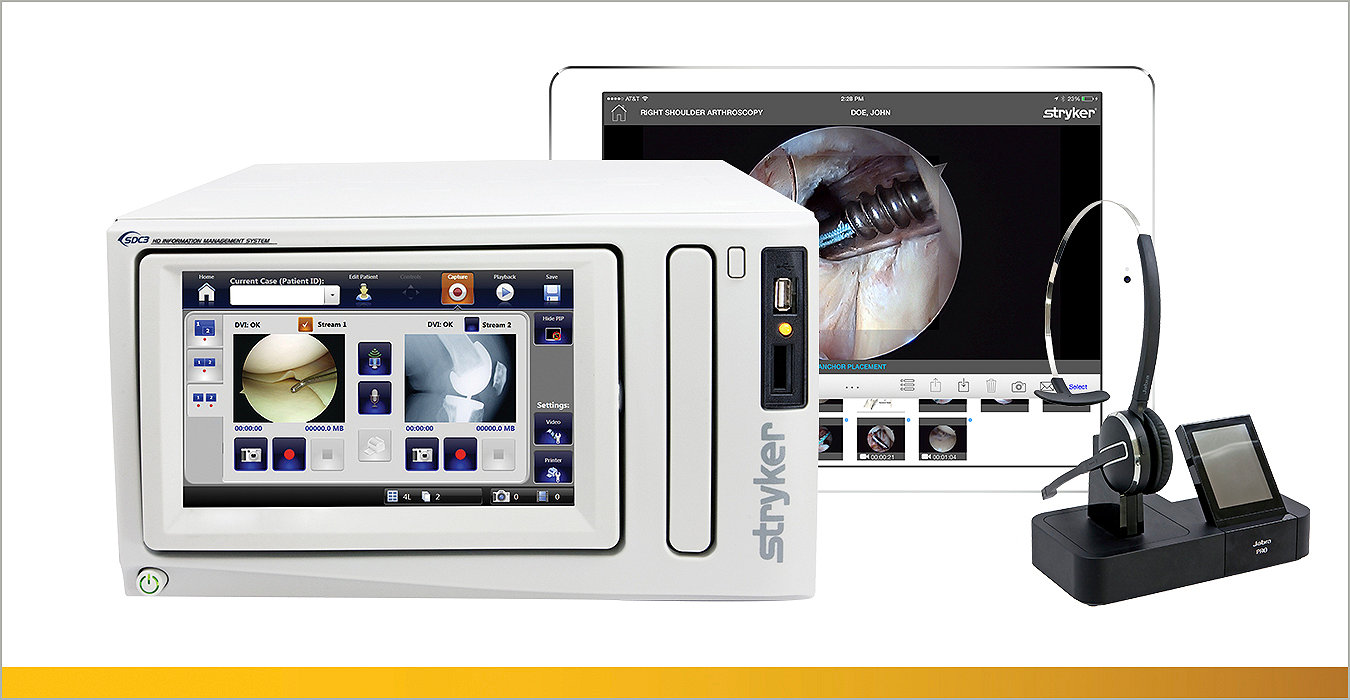
Our innovative sports medicine equipment includes implants, instrumentation, resection and biologic solutions to meet your needs in any repair.
News
and events
and events
Connect with
Sports Medicine
Sports Medicine

Follow Sports Medicine on Twitter and LinkedIn for the latest news and events. Additionally, check out our latest surgical technique videos on VuMedi.
Literature Number: 1000902691 Rev A